
Vaccine Logistics: The key challenges for governments and private sector players

With vaccines historically being developed over a timeline of five to 20 years, the idea that a Covid-19 vaccine could be broadly administered as early as late-2020 is ground-breaking.
In August, Dr. Tedros Andhanom Ghebreyesus, the Director-General for the World Health Organisation, said that WHO has been in extensive consultations to develop a new framework to guide fair and equitable access to Covid-19 diagnostics, therapeutics, and vaccines for all countries.
He added: “We will need to quickly manufacture billions of doses to reach all those who need the vaccine.
“All this means elite planning at the highest levels is needed right now to prepare to vaccinate and treat the world, as new technologies come down the pipeline.”
With over a hundred scientific teams worldwide pushing hard to develop a Covid-19 vaccine, the race is on, with much talk about a vaccine being delivered towards the last quarter of 2020.
But even should they succeed, there are considerable complexities in ramping up and distributing the vaccine, with the pandemic itself still crippling much of our supply and distribution workforce numbers and efficiencies.
For national governments, managing and leading vaccine manufacturers, supply chain logistics and infrastructure adds to their current challenges of containing the pandemic.
WATCH: What Lessons Has Covid-19 Taught On Securing Stable Supply Chains For Future Health Emergencies?
Temperature matters
DHL Customer Solutions and Innovations recently released a white paper titled Delivering Pandemic Resilience, that focuses on securing stable supply chains for essential medical goods during public health emergencies, such as the ongoing Covid-19 pandemic.
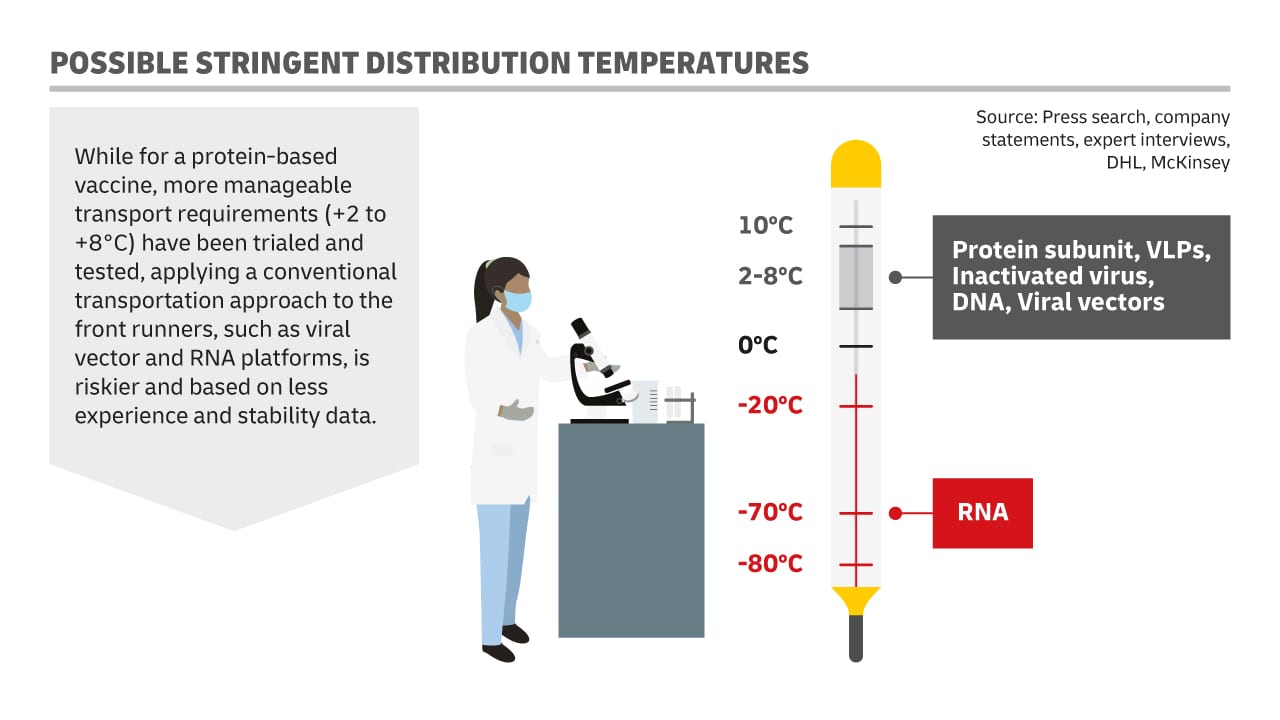
Katja Busch, Chief Commercial Officer DHL and Head of CSI, explains, “When it comes to expected Covid-19 vaccine cold-chain protocols, there are essentially two approaches: the conventional, based on current vaccine protocols; or a more stringent one.”
“For the stringent approach, producers of certain vaccines and their logistics providers have to choose to adhere to strict and extreme temperature requirements (up to -80 °C) to maintain the effectiveness of the vaccines during storage and transport.
“These conditions are in line with the ones used for certain Covid-19 vaccine clinical trials (Phase III) currently.
“These requirements might be lifted over time if vaccine efficacy under higher temperature is proven by stability testing, or if formulations are improved, and additional manufacturing steps are added to increase stability.
“The certainty of success of following a conventional approach partially depends on the vaccine platform. “
While for a protein-based vaccine, more manageable transport requirements (+2°C to +8°C) have been trialed and tested, applying a conventional transportation approach to the front runners, such as viral vector and ribonucleic acid (a polymeric molecule essential in various biological roles in coding, decoding, regulation and expression of genes) platforms, is riskier and based on less experience and stability data.
If things go extraordinarily well, the first vaccine that proves to be clinically effective could, in theory, be the ideal candidate, in that it is not only effective but also allows for scalable production and a manageable distribution at room temperature. However, the announced production capacities are particularly high for vaccines of the RNA and viral vector type.
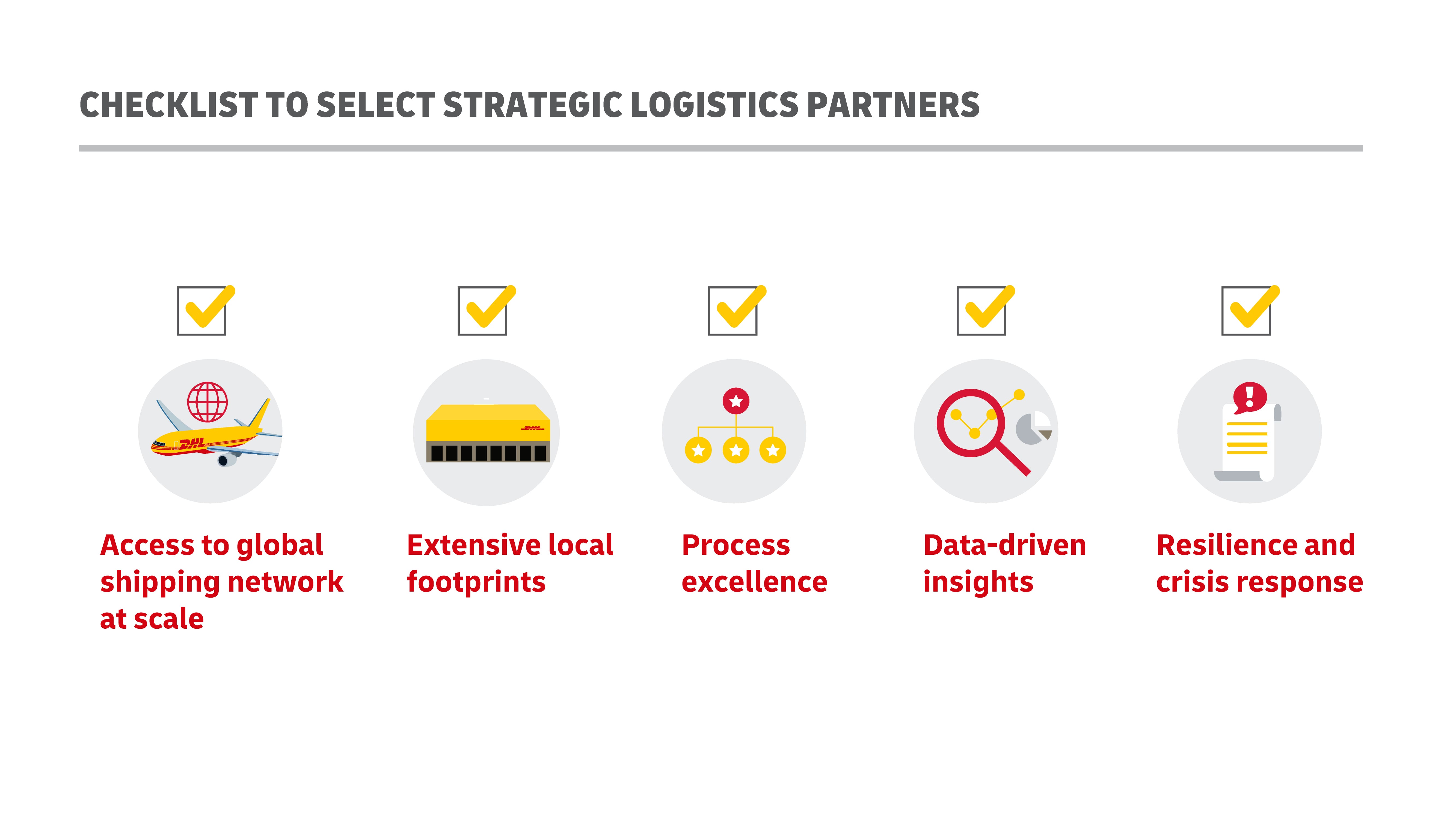
When finished vaccine products are ready for distribution and delivery, most private vaccine companies will work with specialized third-party logistics companies (3PLs). These companies specialize in cross-border movement, customs approvals, maintaining cold chain infrastructure, and equipment to facilitate product pickup and last-mile delivery. They also have access to warehousing, ocean containers, trucks, and other facilities with cold chain refrigeration and temperature measurement capabilities.
Public-private partnerships will be crucial when a vaccine is ready
Under the stringent scenario, pharmaceutical companies, governments, NGOs, and logistics providers need to be prepared to handle these requirements on a scale far beyond the clinical trials.
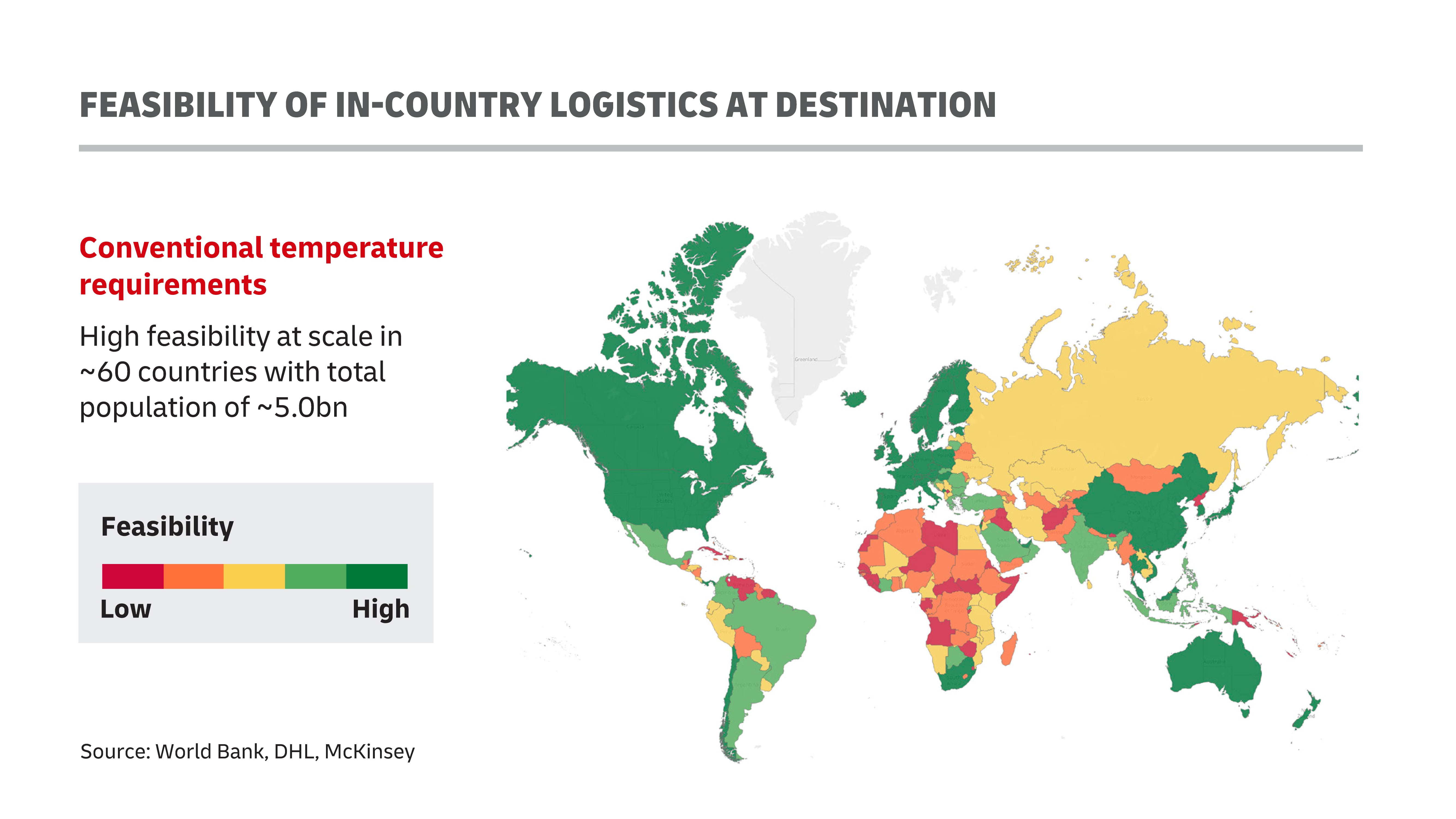
The question is – are national governments ready to take over these vaccines upon their arrival, and manage their distribution channels within their communities with the necessary cold chain facilities? Primary hubs of local distribution could include hospitals and schools, with private-sector workplaces also serving as alternative distribution locales.
Unfortunately, it seems current cold-chain facilities that can accommodate such intensive one-day vaccination events would still only cover a fraction of the healthy population in most, if not all countries.
According to a report by John Hopkins’ Bloomberg School of Public Health, it is highly likely that 70 percent of the global population will need the vaccine for Covid-19 within a short time frame, which increases the importance of a cold chain-ready distribution framework. As it stands, WHO estimates that up to half of all vaccines are wasted each year globally, mostly because of a lack of temperature control and the logistics to support an unbroken cold chain for them.
Dr. Nancy Messonnier, director of the CDC's National Center for Immunization and Respiratory Diseases, also recently raised concerns on cold chain infrastructure and transport in the U.S, according to ABC News.
At a meeting convened by the U.S. Advisory Committee on Immunization Practices, she said, “The complexities of a plan for vaccine storage and handling will have a major impact in our ability to efficiently deliver the vaccine.”
Dr. Erin Fox, senior pharmacy director for drug information and support services at the University of Utah added, “Most pharmacies, most clinics, most places are not going to have ultra-low-temperature freezers on hand.”
Leonora Lim, Vice President in Asia Pacific of DHL Customer Solutions and Innovation, Life Sciences and Healthcare said, “While I am sure all countries have some form of healthcare distribution systems, we still do not know when the vaccines will be ready, and the timing as well as the volume that they will be sent. With differing levels of maturity and sophistication in each country, it has made the need for public-private and public-public partnership more pressing, especially in such a dynamic and volatile environment.”
Referencing the cold-chain public infrastructure in Asia-Pacific, Lim said: “The region is vast, and heterogeneous, including the world’s top two most populous countries – India and China.
Thailand is a positive example when such public-private partnerships go right, according to WHO’s previous case studies on vaccine supply chain and logistics. In 2009, Thailand’s vaccine supply chain and logistics system faced challenges such as wasted and expired vaccine products, inventory control issues, and high costs.
To improve efficiency, Thailand outsourced its vaccine supply chain through a vendor-managed inventory (VMI) system. The system was initially piloted in 28 of 76 provinces and gradually expanded nationwide by late 2010.
The outsourced VMI system streamlined inventory management by reducing from five to three the number of steps that vaccines had to go through in the supply chain. This reduced both the volume of vaccine stock and the length of time vaccines spent in storage.
The economic analysis revealed that in its first year, the VMI system saved nearly one-fifth of the cost of distributing vaccines throughout the national supply chain system. This was achieved through more efficient use of resources, lower logistics costs, and efficiency gains from a reduction of vaccines procured and distributed.
Busch concluded, “Managing such complex supply chains and distribution networks is a demanding task that requires experts to carry them out, and this becomes only more important when talking about highly sensitive products such as vaccines or similar medical products that are crucial to protecting people’s lives.”
“We are convinced that having a go-to logistics partner in place, and most importantly, planning with identified contacts in place ahead of time will go a long way in ensuring stable operations when those are needed most.”
MORE FROM THIS COLLECTION









 English
English